StimFix™ SCS Trial Lead Fixation Device (2)
StimFix™ SCS Trial Lead Fixation Device (2)
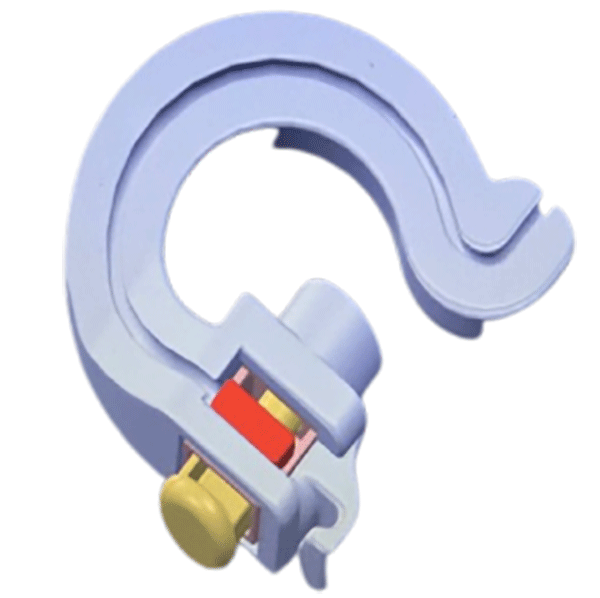
The StimFix™ by Accufix Medical LLC is a sterile, proprietary lead fixation system designed specifically for percutaneous Spinal Cord Stimulation (SCS) trial leads. This innovative, suture-free device offers a standardized, cost-effective solution to prevent trial lead migration, ensuring more accurate assessments of targeted SCS waveforms. Engineered to provide secure fixation for up to 7 days, StimFix is a game-changer in SCS trial lead management, allowing for safer and more consistent outcomes.
StimFix is indicated for use in securing percutaneous SCS trial leads, helping to minimize the risk of lead migration and accidental removal. It is ideal for creating a stable environment for temporary SCS trials, ensuring accurate outcome assessments.
$20.00
In stock
- Suture-Free Application: Avoids the need for invasive sutures, reducing the risk of injury and discomfort.
- Medical-Grade Adhesive: Securely adheres to the patient’s skin for up to 7 days, even under challenging conditions.
- Stability Under Pressure: The proprietary button lock technology ensures that SCS trial leads can withstand up to 15 newtons of force.
- Absorbent Sponge: The silver-impregnated, absorbable sponge helps manage fluid leakage from the insertion site.
- Compact and Comfortable: The small footprint and pliable material allow for easy application and maneuverability, adapting to various skin contours.
- Versatile Positioning: StimFix’s design supports unilateral, bilateral, and ipsilateral lead placements, offering flexibility in use.
- Preparation: Ensure the skin surface is clean and dry before application. Follow healthcare provider instructions for managing the SCS trial lead exit site.
- Application: In the first method, anchor the lead directly onto the skin by tucking it into the inlet. In the second method, feed the lead through the small inlet and glide the StimFix down until it anchors securely on the skin. Confirm lead placement with fluoroscopy, and then secure the remaining lead within the anchoring clip.
- At-Home Care: Inspect the StimFix daily to ensure it remains secure. If the device becomes loose or after 7 days of use, contact your healthcare provider for further instructions.
Precautions and Contraindications
- Sterility: Ensure the device is sterile before use. Do not use if the packaging is opened, damaged, or broken.
- Single-Use Only: Do not reuse, reprocess, or re-sterilize. Reuse may compromise the device’s integrity, leading to potential injury, infection, or cross-contamination.
- Storage: Store in a cool, dry place and avoid using past the expiration date.
- Daily Inspection: Regularly check the device to ensure it is securely holding the SCS trial lead and maintaining its adhesion and overall integrity.
- Allergy Considerations: Do not use on patients with known tape or adhesive allergies.
- Compatibility: Only use with percutaneous SCS leads. Avoid using with other materials.